ILR ECG Analyzer¹
Boost medical staff productivity by focusing on meaningful cardiac arrhythmia eventsReduces the number of false positives by 79%²
Maintaining a sensitivity of 99%²
Driven by new indications (cryptogenic stroke, syncope, etc.), by recent technological progress and by the development of remote monitoring, the implantation of ILRs (Implantable Loop Recorders) continues to increase. The number of implanted patients is expected to double in the next 10 years.
The ILR, an essential medical device for detecting arrhythmias, however, emits a high number of false-positive episodes, which makes remote monitoring particularly time-consuming.
To make your remote monitoring activity easier, Implicity has launched a new artificial intelligence algorithm: ILR ECG Analyzer. This software medical device reduces the number of false positives by 79% when analyzing ECG recordings from patients implanted with Medtronic ILRs, while maintaining a sensitivity of 99%.²
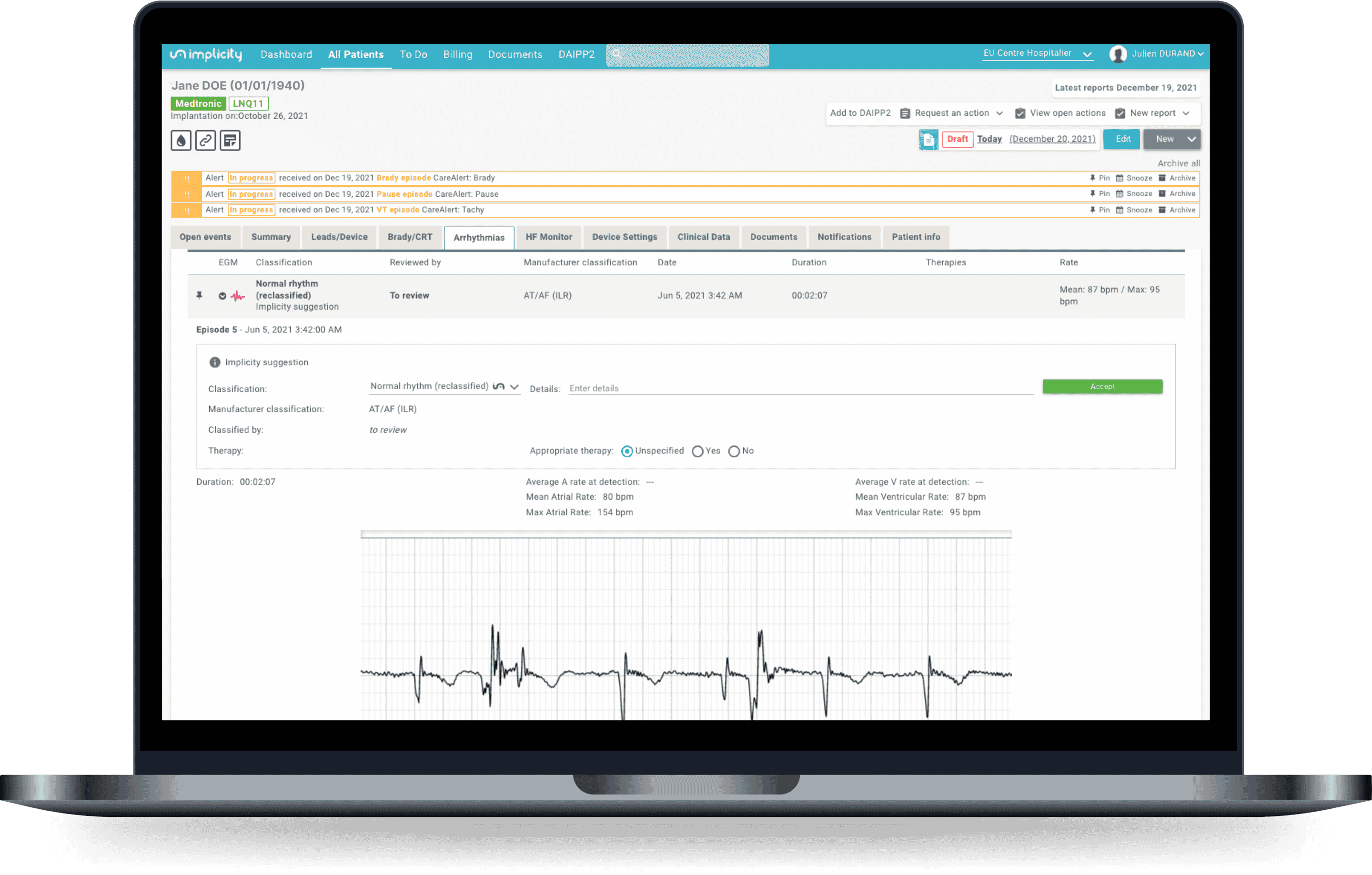
An episode classified as bradycardia by the ILR is identified as a normal rhythm by ILR ECG Analyzer.
The benefits for remote monitoring teams:
Improved arrhythmia detection, allowing to focus on real events
Significant time savings, allowing more patients to be monitored
¹IM007: 2021. ILR ECG ANALYZER created by Implicity is intended to be used by qualified healthcare professionals for the assessment of arrhythmias in Insertable Cardiac Monitor (ICM) ECG data. ILR ECG ANALYZER supports downloading and analyzing data recorded in compatible formats from ICMs. This version of the ILR ECG ANALYZER only supports ECG data from Medtronic ICMs. ILR ECG ANALYZER is intended to be electronically interfaced with other computer systems (remote monitoring platforms) that supply the ECG data to ILR ECG ANALYZER and receive the output of ILR ECG ANALYZER (analysis) for viewing by healthcare professionals. ILR ECG ANALYZER provides ECG signal processing and analysis, to detect asystole, bradycardia, atrial tachycardia or atrial fibrillation, ventricular tachycardia, normal rhythm and artifacts. ILR ECG ANALYZER is not for use in life supporting or sustaining systems or ECG monitor and Alarm devices. ILR ECG ANALYZER interpretation results are not intended to be the sole means of diagnosis. It is offered to physicians and clinicians on an advisory basis only in conjunction with the physician’s knowledge of ECG patterns, patient background, clinical history, symptoms, and other diagnostic information. ILR ECG ANALYZER is interfaced with the compatible remote monitoring platform from which it receives compatible ICM data file input (Medtronic LNQ11 Medtronic REVEAL XT 9529 Medtronic REVEAL DX 9528) and to which it transmits the output. Read carefully all instructions before use. Results: sensitivity: 98.64 % (509/516), false positive rate: 24.03 % (68/283). FDA cleared Class II medical device and CE marked Class I medical device (under medical device directive 93/42).
²https://academic.oup.com/eurheartj/article/42/Supplement_1/ehab724.0316/6393406?login=true
