Advanced Research Tool
Are you looking to drive clinical studies and improve the standard of care?
Harness the full power of data with the Implicity platform for research!
Implicity can assist you in collecting real time data from all cardiac implantable electronic devices, conducting clinical studies, and exploring new research fields. From bringing a tailored eCRF to organizing cross-device data, we cover all aspects of your remote cardiac monitoring activity.
Embrace the latest Remote Monitoring technology tailored for research
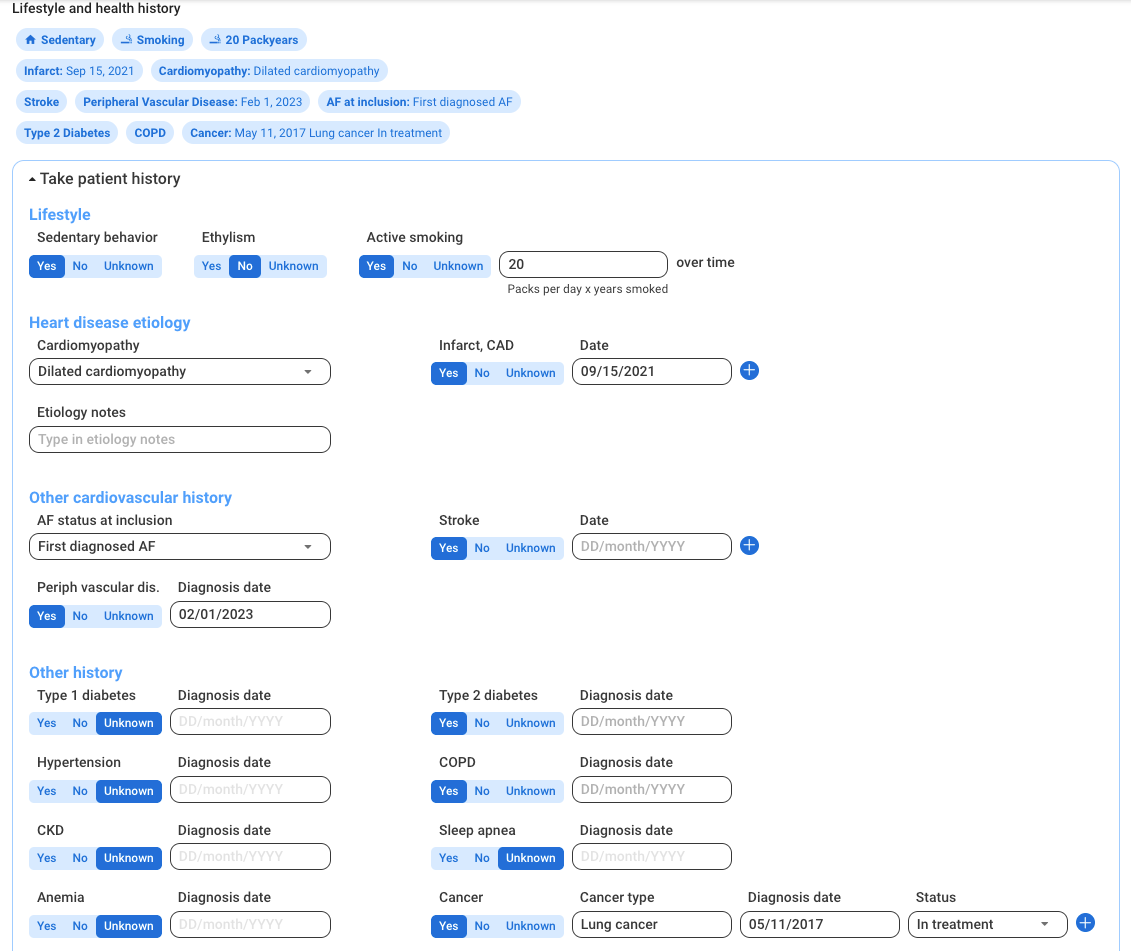
Raw data readily available for research
Normalized patient data across manufacturers
Centralized multi-hospital network data to build scalable research projects
Customized eCRF
Patient consent tracking
Implicity was selected as the official remote monitoring platform of one of the largest European research consortium on ICDs, DAI-PP2.

Learn more about DAI-PP Registry
Implicity is the official remote monitoring platform of one of the largest European research consortium on ICDs.
DAI-PP – one of the largest European research programs on ICDs
DAI-PP is a consortium of 17 Universities and private hospitals in France. Started in January 2020, it aims for a massive real-time follow-up of patients with ICD. This prospective annual follow-up is implemented to test different hypotheses using latest technologies and multidisciplinary expertise and addressing high-profile research fields such as big data, remote monitoring, genetics…
- 5,000+ patients expected to be enrolled during the first two years of the Program;
- 1400+patients already enrolled;
- 17 French university hospitals and private clinics
- Research program supported by the French Society of Cardiology, the French National Institute of Health and Medical Research (INSERM) and Clinique Pasteur;
- Long-term ambition (beyond the initial 5 year period)
- Consolidated data research from pilot phase, issued in 2019 – Serge Boveda & al.
- Implicity is the official remote monitoring platform used by the consortium
Implicity allows to use all raw data readily available for research within one single platform. With data collection from all manufacturers, patient consent management, and an eCRF available online, the platform is the turnkey solution for research.
